PCB Cleanliness Standards: What Are the Functions of It?
By:PCBBUY 04/07/2022 09:48
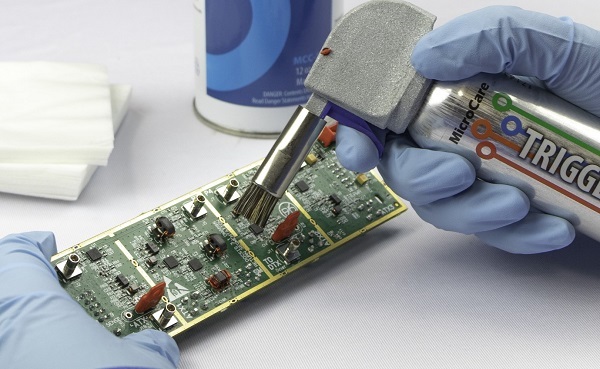
A circuit board goes through many different processes while it is being manufactured, from the etching of the metals during fabrication to the application of solder during the assembly. All of these processes have the potential of leaving various residues and contaminants on the finished board. These residues can cause short-term and long-term problems with the board.
In the short term, any visible residues and contaminants on the board will be very obvious and could cause concern, even in cases in which it may not actually be a problem. No-clean flux residue is an example of this. Although there aren’t any problems with it, it will leave the board looking as if it hasn’t been properly cleaned. For cleanable fluxes that aren’t properly removed during the cleaning process, their residue could cause problems in testing. They may also cause intermittent functional problems in the board that are difficult to find or reproduce.
In this passage, we will provide all the details of PCB cleanliness standards please check and read the content below for more information.

What are the standards of PCB cleanliness?
The PCB standard that directly addresses cleanliness for board fabrication is IPC-5704 Cleanliness Requirements for Unpopulated Printed Boards, which covers preventing or removing ionic contamination and other types of unwanted debris. IPC-6012D, which extends to include PCBAs, covers cleanliness as well as all aspects of circuit board manufacturing. For most PCBA applications, the rules and guidelines of these standards are sufficient. However, the requirements for medical device electronics boards are much higher. In fact, for these boards, a cleanliness regimen is crucial and should include validation that sufficient levels of contaminants are removed during the process.
When it comes to PCB cleanliness standards for medical device electronics there is almost universal agreement on two points.
The need for post-assembly cleaning of circuit boards is “critical” to ensure that medical electronic devices function properly and meet regulatory requirements for reliability.
A specific standard that directly and comprehensively addresses the cleanliness of PCBAs used in medical device electronics is lacking.
The rapid expansion of the PCBA industry, especially in terms of board complexity and advanced functionality has presented a significant challenge for regulatory bodies to update or create new rules and guidelines that are pertinent. As a result, much of the attention has gone to management and administrative requirements instead of technical specifications. This has allowed the identified issue of the absence of a targeted PCB cleanliness standard to go unaddressed.

Adherence to IPC-5704
The development of all medical devices is required to adhere to a specific set of quality control standards. The set of standards that specifically go over cleanliness requirements for unpopulated printed circuit board fabrication is known as IPC-5704. A large portion of this goes over the necessary steps for preventing and removing any sort of ionic contamination or other unwanted debris from a printed circuit board.
Ultrasonic Cleaners
As far as cleaning methods for a PCB assembly manufacturer goes, this method is the absolute best. This is because ultrasonic cleaning involves a temperature-controlled process that is completed at set time intervals. But of course, the solution that is used during this process is going to impact just how effective this method ends up being.
Automatic Washers
This process involves putting PCBs into baskets and then dipping them into a solution. Once they are removed from the solution, they are then dried off. But it is important to select a good solution so that none of the printed circuit board assembly materials suffer any damage.
Manual Cleaning
Although used a lot less commonly, there is also the option to clean a printed circuit board fabrication by using a manual method. The problem with this method is that it becomes nearly impossible to reach some of the areas, particularly on smaller PCBs. However, for certain components like microphones, the manual method is the only one that can be used.
What are PCB cleaning and testing methods?
To clean circuit boards, PCB manufacturers will use an in-line wash with heated DI (deionized) water. The water pressure will be set according to the guidelines set by the solder and equipment manufacturers. There may also be additional cleaning processes, including IPA (isopropanol alcohol) wipedowns, removal of no-clean flux residues, and ultrasonic cleaning. These specialized cleanings are typically done only when there are documented requests for them.
To ensure that they are performing adequate cleaning of PCBAs, CMs should regularly test their cleaning processes. This is usually done with the industry standard ROSE (Resistivity of Solvent Extract) test. The CM will submit a sample for regular testing, usually to an outside service provider. The CM will also visually inspect the finished PCBA to make sure that it passes their internal standard of cleanliness.
Industry Category