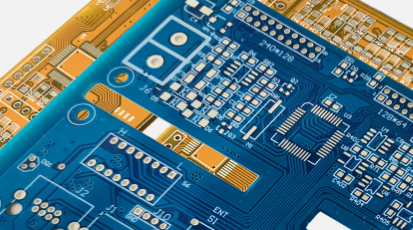
What Are the Main Effective Traceability Processes during PCB Manufacturing?
By:PCBBUY 08/30/2023 15:57
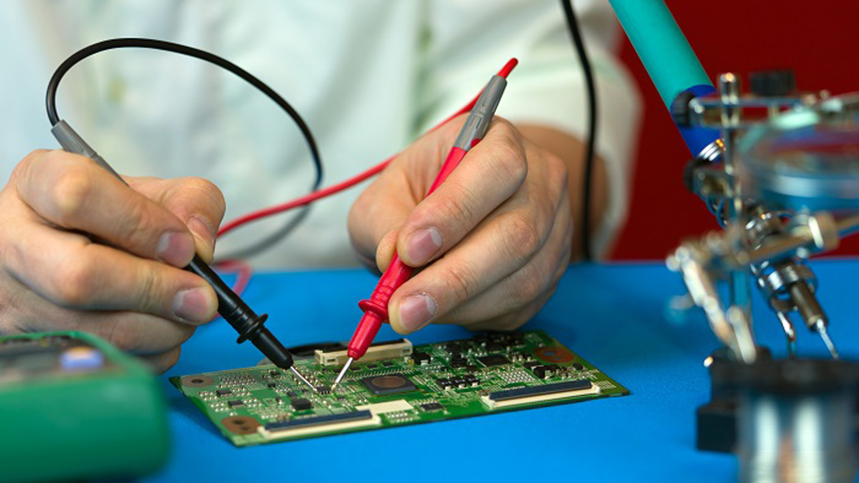
Being able to account for every possible variable is the key to traceability. This need is especially true in the medical equipment field, where every piece of the assembly must be able to be tracked back to its source. Implementing this traceability constraint is an enormous task, particularly when building a printed circuit board, as several components and materials go into manufacturing a PCB.
To achieve traceability in medical devices, it is crucial to incorporate a traceability matrix specifically designed for medical devices. This traceability matrix, also known as the “traceability matrix medical device,” enables the comprehensive tracking and documentation of the origin and journey of the building blocks of the medical device through the supply chain. By utilizing this traceability matrix, manufacturers can ensure compliance with regulatory standards and maintain transparency throughout the manufacturing process.

Medical Devices and The Need for Transparency
A PCB contract manufacturer must be able to fulfill the traceability requirements for the medical device in production. The specifics of the board will determine which industry standard, such as ISO 13485, must be met. To fulfill these requirements, a CM should understand and be fully certified in the standards.
Documentation is the cornerstone of traceability: if there is a problem with a part or product, it will be easy to identify any other parts or products that might be similarly affected. Accurately identifying parts and products is fundamental to tracking, saving time and money in the event of a supply chain investigation. Agencies strictly enforce precise product identification, and a CM must have a robust identification system (along with their tracking system) to quickly identify what parts are in use.
This identification should include compliance certifications, test results, and other crucial electronic product data. Since many PCBA or subsystems appear similar but are configurable in either hardware or software, their performance can vary significantly and call for precise documentation. Thorough documentation can avoid numerous performance or reliability problems in the future. Traceability also aids design teams as they continue to develop and improve the product; the better the tracking of the parts, the richer the performance data will be for analysis.
Traceability Processes During PCB Manufacturing
Achieving traceability requires the work of a dedicated contract manufacturer with processes in place to accurately identify and track the information under consideration. A good CM will have a work order documentation system for building circuit boards; this serial number on the completed board can then trace back to the original work order. The documentation system in use should include the following work order information:

Purchase order (PO) data: The work order should contain all of the POs used for procuring the parts and materials for the PCBA. The work order will include the unassembled PCB manufacturing from the fabricator, components, sheet metal, cables, and other manufacturing materials. This data should also include supplier data codes when possible.
Manufacturing process steps: It is also helpful for traceability to know the manufacturing steps taken during assembly, including inspection and testing.
Manufacturing technicians: Knowing which technicians performed specific operations also support traceability. The technician in question can then field any questions on a PCBA.
An intricate tracking system relies on multiple sub-processes for effectiveness. Documentation must be in place, along with a method to assign and match up tracking numbers to products and parts the CM is using. Then, there must be the ability to identify those products and parts, usually with a barcode tracking system. The traceability matrix medical device plays a crucial role in this process, enabling the systematic identification and tracking of products and parts throughout the assembly of medical devices. Finally, there has to be a database that sorts all of this data, including the information captured by the traceability matrix medical device, and presents it in an organized and easy-to-understand format.
An intricate tracking system relies on multiple sub-processes for effectiveness. Documentation must be in place, along with a method to assign and match up tracking numbers to products and parts the CM is using. Then, there must be the ability to identify those products and parts, usually with a barcode tracking system. Finally, there has to be a database that sorts all of this data and presents it in an organized and easy-to-understand format.
Industry Category